Detection of Tetracycline Resistance Gene (TetA) in Bacterial Isolates from Chicken Meat Samples in Bhopal, Madhya Pradesh
Manisha Shukla 1, Anjali Tomar 1, Naeem Akhtar1, Rohit Kumar Vishwakarma 2,
Vol 1/Issue 4
Abstract
The global rise of antimicrobial resistance (AMR) poses a serious threat to public health, limiting treatment options for infectious diseases and compromising the effectiveness of antibiotics. While the clinical misuse of antibiotics is a known contributor, the widespread use of antibiotics in animal agriculture has emerged as a significant driver of resistance. This study investigates the presence of antibiotic-resistant bacteria, particularly those carrying the TetA gene responsible for tetracycline resistance, in chicken samples collected from local vendors in Bhopal, Madhya Pradesh.
Bacterial isolates were obtained from raw chicken meat samples and subjected to DNA extraction using the phenol-chloroform method. Polymerase chain reaction (PCR) was performed using universal primers to detect the presence of the TetA gene. Additionally, the study aimed to standardize a real-time quantitative PCR (qPCR) protocol for the rapid and sensitive detection of antibiotic resistance genes in foodborne bacterial populations.
The findings of this study are expected to provide critical insights into the prevalence and spread of antibiotic resistance in the food supply chain. By identifying resistant bacterial strains and optimizing detection methods, this research contributes to the broader efforts to monitor and control antimicrobial resistance in India and globally.
Introduction
Antimicrobials have historically been one of the most potent forms of chemotherapy, drastically reducing deaths from infectious diseases. Evidence shows that exposure to antibiotics predates the modern era, with traces of tetracycline found in ancient human remains. This suggests that antibiotic resistance has a long history, with resistance genes existing in nature long before the clinical use of antibiotics. Resistance has evolved through mechanisms like chromosomal mutations, and many resistance genes have ancient origins, dating back millions or even billions of years. Antibiotics, derived from the Greek words for “against life,” are compounds used by microbes for various purposes, including competition and signaling.
Despite their life-saving role, the rise of antibiotic-resistant bacteria poses a significant threat to global health. Resistance has emerged rapidly, with bacteria evolving to withstand multiple drugs. This problem is exacerbated by the lack of new antibiotics in development, making it difficult to treat multidrug-resistant organisms like MRSA and VRE. The first quinolone, nalidixic acid, highlighted the potential of these drugs, but the emergence of resistance has limited their effectiveness.
Antibiotics have significantly reduced deaths from infectious diseases and increased life expectancy, but the spread of resistance now challenges these achievements. Research has primarily focused on pathogens, and antibiotic resistance genes have been found in environments contaminated by human and animal waste, highlighting the role of sewage systems in spreading resistance. The World Health Organization identifies antibiotic resistance as one of the top threats to public health in the 21st century, with resistant bacteria and genes frequently found in aquatic environments and linked to human activities.
Efforts to combat this issue must include improved sanitation, better management of antibiotic use, and the development of new treatments. The rapid spread of resistance genes, driven by environmental pollution and the overuse of antibiotics, underscores the urgent need for global action to preserve the effectiveness of antimicrobial treatments and protect future generations.
The resistance to antibiotics, which has spread around the world as a result of careless and unregulated use, makes treating and managing bacterial illnesses like Salmonella infections challenging. Both phenotypic techniques, such as the disk diffusion method, and genotypic techniques, which show the presence of genes generating resistance, are used to examine antibiotic resistance levels. MDR, or multiple-drug resistance, in varieties of Salmonella is known to have zoonotic origins, meaning that consuming contaminated goods can spread the infection from animals to people. As a result, identifying the profiles of antibiotic resistance in field isolates is crucial for updating pertinent laws and creating more potent national antibiotic resistance prevention plans (Arkali, A., & Çetinkaya, B. 2020).
The resistance to antibiotics, which has spread around the world as a result of careless and unregulated use, makes treating and managing bacterial illnesses like Salmonella infections challenging. Both phenotypic techniques, such as the disk diffusion method, and genotypic techniques, which show the presence of genes generating resistance, are used to examine antibiotic resistance levels. MDR, or multiple-drug resistance, in varieties of Salmonella is known to have zoonotic origins, meaning that consuming contaminated goods can spread the infection from animals to people. As a result, identifying the profiles of antibiotic resistance in field isolates is crucial for updating pertinent laws and creating more potent national antibiotic resistance prevention plans.
Antibiotics present in the environment put soil microorganisms under selective pressure to develop antibiotic resistance, which can lead to the formation of resistant microorganisms and genes that resistance to antibiotics (ARGs). ARGs are emerging pollutants that can remain in the atmosphere even after the selective force that caused their formation has been eliminated, regardless of whether or not they are found in living or dead cells. ARGs are moveable and can spread between microorganisms, including pathogenic and non-pathogenic bacteria, by integrating into a variety of movable genes, like plasmids or transposons (Zhao, X.,et.,al 2017).
Multiple resistant drugs (MDR) in bacteria is developing, reflecting the growing resistance to widely used antimicrobial medicines. This growing resistance poses a significant threat to the health of humanity. The emergence and spread of resistant bacteria have been connected to the use used antimicrobial substances in animal husbandry. For instance, handling or eating meat tainted with infections can spread resistant germs from chicken products to humans. Commensal bacteria, on the other hand, are a reservoir and a carrier of factors determining resistance in the environment, making their resistance equally significant.
Cross-resistance including the selection of resistant antibiotic genes (ARGs), which may move laterally on mobile genetic parts (MGEs) via the process of horizontal gene transfer (HGT), might result from exposure to antimicrobials of various classes. Genes can be passed between creatures of the same species or different species, as long as they maintain a close ecological interaction. This occurrence is known as horizontal gene transfer (HGT) (Racewicz, P.,et.,al 2022).
One of the biggest issues facing human health today is antibiotic resistance, and some argue that we are perilously near to returning to a time before the invention of antibiotics. There is mounting evidence and worry that the use of antibiotics in agriculture may be a major factor in the rising rates of resistance to antibiotics. Most antibiotics are used on livestock in many nations; in the US and many other countries, most antibiotics are used as preventative measures or to promote growth. Antibiotics used in both human and cattle therapy, including third-generation cephalosporins, are particularly concerning; nonetheless, the use of different antibiotics alone carries the potential of co- or cross- selection of a resistance to human medicines.
Vancomycin-resistant Enterococci is one of the important antibiotic-resistant human diseases that have been reported to be reservoired in livestock populations. Antibiotic usage in livestock is unrestricted in many other nations and a large portion of the developing globe, and prescribed is not even needed for human use. Africa generally allows unfettered use of antibiotics in animals. As a result, there is a chance to compare antibiotic resistance trends among cattle from various parts of the world in order to get insight into the overall consequences of antibiotic usage practices, which vary greatly across the globe. Specifically, little is understood about the level of antibiotic resistance in African farm animals or possible connections to human disease, despite the fact that significant efforts have been made to improve the abysmal conditions of public health in the continent (Olonitola, O. S.,et.,al 2015).
Poultry is currently one of the primary sources of meat consumed around the world. Only in Germany were over 800,000 tons of chicken processed in the initially half of 2020. Water is used in the operations of processing, cleaning, and sanitizing chicken production facilities. Processing requires anywhere from 5,000 to 21,000 L of liquid per ton of meat, depending on the method used for slaughter. This causes large volumes of wastewater to form along the chain of slaughter, and this effluent typically contains a variety of enteric infections. Slaughterhouse effluent has a significant organic concentration because of solved fibers, amino acids, and lipids in addition to microbial burdens (Savin, M.,et.,al 2021).
When antibiotics are used on food animals, there is a risk that resistant bacteria will develop and proliferate. Many nations have limited or outlawed the use of some antibiotics in food animals in an effort to lessen their harmful effects. These restrictions apply particularly to antibiotics that are highly important for human use or are used for nontherapeutic purposes. Third-generation cephalosporin use is prohibited in Australia, and the use of fluoroquinolones in food animals has never been permitted. Chlortetracycline is approved for use in poultry to treat egg- producing chickens (layers), while a number of antibiotics, including oxytetracycline, amoxycillin, neomycin, lincomycin, and spectinomycin, are approved for treatment in meat-producing chickens (broilers). Zinc-bacitracin and Virginiamycin are permitted for the treatment and prevention of necrotic enteritis (Liu, Y.,et.,al 2020).
Aims and objectives
To identify the bacterial species present in chicken samples obtained from various chicken vendors in Bhopal, Madhya Pradesh.
- To extract DNA from isolates by employing the phenol chloroform technique, Using universal primers, perform PCR for the antibiotic-resistant gene (Tet A).
- To standardize the quick detection of antibiotic resistance genes using qPCR in samples prepared from chicken materials.
Review of literature
Antimicrobial resistance (AMR) has emerged as a significant global health crisis over recent decades, with various international health organizations highlighting its severity. This resistance has been described as a global pandemic, major public health hazard, and a potential catastrophe that could reverse the gains made in combating infectious diseases. Mortality projections indicate that infections could become as deadly as they were a century ago due to rising resistance. The continuous development of resistance to new antibiotics underscores the relentless battle between medical innovation and bacterial adaptation. This has led to concerns about the future efficacy of antibiotic treatments.
The identification of methicillin-resistant Staphylococcus species through multiplex PCR highlights the ongoing need for advanced molecular diagnostics to detect and manage resistant strains effectively. The introduction of any new antibiotic inevitably leads to the development of resistance, driven by the selective pressure of antibiotic use. This pattern has been observed repeatedly, leading to an ongoing cycle of antibiotic development and resistance emergence.
Human antibiotic consumption plays a significant role in the development of resistance. There is a strong correlation between antibiotic use and the prevalence of resistant infections. Misuse of antibiotics, such as taking them for viral infections or not adhering to prescribed regimens, exacerbates the problem. Educating patients about the appropriate use of antibiotics and ensuring compliance with treatment plans are crucial steps in combating resistance. The use of empirical antibiotic prescriptions without precise diagnostic information also contributes to the spread of resistance. Developing rapid ABR (antibiotic resistance) profiling tests could help in prescribing the most effective drugs, reducing the likelihood of treatment failure due to resistance. Bacteria can develop resistance through intrinsic mechanisms, such as structural or functional properties that inherently protect them from certain antibiotics. For instance, the biocide triclosan is ineffective against Pseudomonas species due to their inherent resistance mechanisms, including the presence of an insensitive enzyme target.
Molecular approaches are essential for the rapid and accurate identification of pathogenic bacteria. Techniques like RAPD analysis and 16S rRNA gene sequencing are commonly used to distinguish between bacterial species and identify specific strains. The comprehensive sequencing of the 16S rRNA gene and analysis of other genes like gyrB offer precise methods for microbial speciation. The development of databases such as the Antibiotic Resistance Genes Database (ARDB) provides valuable resources for tracking and understanding resistance genes, covering a wide range of antibiotics and resistance mechanisms.
Efforts to combat AMR require a multifaceted approach, including the development of new antibiotics, improved diagnostic techniques, better patient education, and more stringent regulation of antibiotic use. The rapid spread of resistance genes, fueled by human activity and environmental contamination, highlights the urgent need for global action to preserve the effectiveness of antibiotics and safeguard public health.Drug- resistant bacteria are becoming more prevalent worldwide, which has an adverse impact on human health and animal welfare. The evaluation of resistance factors in connection to the phenotypic resistance of E. Coli isolates found in various ecological settings has been the main focus of our research. The samples were taken from the neck skin, excrement, and litter of chickens. (Racewicz, P et.,al 2022). The European Union (EU) and other regulatory bodies have banned the use of antibiotics in poultry and other animals meant for human consumption. This includes the use of antibiotics as growth promoters and as a last resort to stop the spread of Gram-negative bacteria that are resistant to multiple antibiotics. The demand for better food monitoring is growing due to the unstoppable rise in antibiotic resistance. Gram negative bacteria were identified from retail chicken integrase genes using selective medium. The Gram negative population in the samples was qualitatively analyzed using Denaturing Gradient Gel Electrophoresis (DGGE), which revealed the anticipated diversity based on DNA sequencing and band stabbing. (McNeece, G.,et.,al 2014).In the production of broiler chickens, growth-promoting agents (GP) such as virginiamycin (VG) and zinc bacitracin (ZB) are utilized as antibacterial agents. This study set out to assess how the use of ZB and VG affected the development of antibacterial resistance in a commercial broiler chicken farm (Thibodeau, A,et.,al 2008).
It is unclear how worldwide norms on the use of antibiotics in cattle contribute to the emergence of bacteria resistant to antibiotics. Few statistics are available from African countries, where the prevalence of antibiotic-resistant diseases in humans is considerable. Given that the patterns of antibiotic resistance among pathogen-containing genera suggest the possibility of antibiotic treatment failure, chicken litter linked to antibiotic usage and agricultural techniques may pose a risk to public health. But overall, the MARI values were lower than those for Escherichia coli from very intensive chicken operations in developed countries (Olonitola, O. S.,et.,al 2015).
Cattle, pigs, or poultry excrement easily yields microorganisms resistant to antibiotics. It’s unclear, though, if the production of certain farm animals is linked to a higher or lower incidence of antibiotic resistance. Therefore, in this investigation, we employed real-time polymerase chain reaction (PCR) to quantify the prevalence of antibiotic genes in DNA extracted from farm animal feces. Therefore, compared to laying hens, cattle and pig production systems may provide a more significant reservoir of antibiotic-resistant microorganisms (Faldynova, M.,et.,al 2013).
Numerous investigations on the presence of antibiotic resistance genes (ARGs) in animal-associated bacterial populations are the outcome of growing concern over the use of antibiotics in food production. The prevalence of ARGs on farms where hens are kept intensively with little to no antibiotic usage is not well- documented. This work surveyed two antibiotic-free chicken farms for the presence of ARGs and mobile genetic elements known to promote the spread of ARGs using a high-throughput quantitative PCR array. Before this study, the study farms had not used antibiotics for five years. Without any direct selecting pressure, the findings establish a baseline for the prevalence of resistance genes in the chicken production system (Liu, Y.,et.,al 2020).
Materials and methods
Sample collection: Around 10 Chicken samples were collected from different places which comprehensively used by Human. Collected samples were inoculated on Nutrient Agar media. Random colonies of bacteria were picked and seeded in the LB broth and after 24 hours we centrifuge and got pallet, which were further used for DNA extraction.
Genomic Dna Extraction From Bacteria Using Phenol Chloroform Method
The isolation and purification of DNA from cells is one of the most common procedures in contemporary molecular biology and embodies a transition from cell biology to the molecular biology (from in vivo to in vitro). The isolation of DNA from bacteria is a relatively simple process. The organism to be used should be grown in a favorable medium at an optimal temperature, and should be harvested in late log to early stationary phase for maximum yield.
The genomic DNA isolation needs to separate total DNA from RNA, protein, lipid, etc. Initially the cell membranes must be disrupted in order to release the DNA in the extraction buffer. SDS (sodium dodecyl sulphate) is used to disrupt the cell membrane. Once cell is disrupted, the endogenous nucleases tend to cause extensive hydrolysis. Nucleases apparently present on human fingertips are notorious for causing spurious degradation of nucleic acids during purification. DNA can be protected from endogenous nucleases by chelating Mg2++ ions using EDTA. Mg2++ ion is considered as a necessary cofactor for action of most of the nucleases. Nucleoprotein interactions are disrupted with SDS, phenol or proteinase K. Proteinase enzyme is used to degrade the proteins in the disrupted cell soup. Phenol and chloroform are used to denature and separate proteins from DNA. Chloroform is also a protein denaturant, which stabilizes the rather unstable boundary between an aqueous phase and pure phenol layer. The denatured proteins form a layer at the interface between the aqueous and the organic phases which are removed by centrifugation. DNA released from disrupted cells is precipitated by cold absolute ethanol or isopropanol.
Required chemicals:
LB Broth
Bacterial culture
Reagents
TE buffer (pH 8.0)
10% SDS
Proteinase K
Phenol-chloroform mixture
5M Sodium Acetate (pH 5.2)
Isopropanol
70% ethanol
Autoclaved Distilled Water
Eppendorf tubes 2 ml
Micropipette
Microtips
Preparation of reagents
- TE BUFFER (pH 8.0): 10 mm Tris HCl (pH 8.0), 1 mm EDTA (pH 8.0).
- 10% SDS: Dissolve 10 g of SDS in 100 ml autoclaved distilled water.
- PROTEINASE K: Dissolve 10 mg of Proteinase K in 1 ml autoclaved distilled water.
- PHENOL CHLOROFORM MIXTURE: The pH is very important. For RNA purification, the pH is kept around pH 4, which retains RNA in the aqueous phase preferentially. For DNA purification, the pH is usually 7 to 8, at which point all nucleic acids are found in the aqueous phase. Mix equal volume of phenol with chloroform. Keep the mixture on ice and add 20 ml TE buffer, extract by shaking for 15 minutes. Remove the dust on the surface layer using a pipette. Repeat 4-5 times. Add 30-40 ml of TE buffer and store it on ice.
- 5M SODIUM ACETATE: Dissolve 41 g of sodium acetate in 100 ml distilled water and adjust pH with dilute acetic acid (pH 5.2).
- ISOPROPANOL
- 70% ETHANOL
Procedure:
- Two ml overnight culture is taken and the cells are harvested by centrifugation for 10 minutes.
- 875 µl of TE buffer is added to the cell pellet and the cells are resuspended in the buffer by gentle mixing.
- 100 µl of 10% SDS and 5 µl of Proteinase K are added to the
- The above mixture is mixed well and incubated at 37° C for an hour in an incubator.
- 1 ml of phenol-chloroform mixture is added to the contents, mixed well by inverting and incubated at room temperature for 5 minutes.
- The contents are centrifuged at 10,000 rpm for 10 minutes at 4°
- The highly viscous jelly like supernatant is collected using cut tips and is transferred to a fresh tube.
- The process is repeated once again with phenol-chloroform mixture and the supernatant is collected in a fresh tube.
- 100 µl of 5M sodium acetate is added to the contents and is mixed gently.
- 2 ml of isopropanol is added and mixed gently by inversion till white strands of DNA precipitates out.
- The contents are centrifuged at 5,000 rpm for 10 minutes.
- The supernatant is removed and 1ml 70% ethanol is added.
- The above contents are centrifuged at 5,000 rpm for 10 minutes.
- After air drying for 5 minutes 200 µl of TE buffer or distilled water is added.
- 10 µl of DNA sample is taken and is diluted to 1 or 2 ml with distilled water.
- The concentration of DNA is determined using spectrophotometer at 260/280 nm. a
- The remaining samples are stored for further experiments.
Precautions:
1 Cut tips should be used so that the DNA is not subjected to mechanical disruption 2 Depending on the source of DNA the incubation period of Proteinase K should extended. 3 The phenol chloroform extraction should be repeated depending on the source of DNA to obtain pure DNA.
4 DNase free plastic wares and reagents should be used.
POLYMERASE CHAIN REACTION (PCR)
Polymerase chain reaction (PCR) is a powerful tool that allows the amplification of DNA. Since its development, advances in technology have allowed wider applications of its use, incorporating whole genome amplification, real time amplification and amplification from RNA samples. PCR typically involves three steps: denaturation of double stranded DNA, annealing of oligonucleotide primers and extension of a duplicate strand of DNA; which are repeated for about 35 cycles. Denaturation of the double stranded DNA occurs by heating the sample to 95°C, following this DNA is cooled to about 50- 65°C so that primers can anneal to the now single stranded DNA at positions flanking the region of interest. These primers are typically about 20bp long to allow for specificity of a region and are complementary to the DNA with one primer binding to the 5’end and one binding to the 3’end of the region of interest. Following annealing of the primers, the reaction is heated to 72°C and deoxynucleotides are added to the ends of the primers so that a new strand of DNA is extracted. These nucleotides (dNTPs) are incorporated into the growing strand of DNA by a polymerase, just as they would during DNA replication. Specifically, in PCR Taq polymerase is used for this step as it is thermostable and is still active after heating to high temperatures. Also included in a PCR reaction is a buffer containing various salts including magnesium chloride, which aid in the process. The denaturation, annealing, extension cycle is completed about 25-40 times so that the number of DNA fragments of interest grows exponentially. That is, after the first cycle there will be2 copies, after the second cycle4, after the third cycle 8 and so on until you would end up with 33.5 million copies after 25 cycles.
Methods:-
NB: PCR is based upon the amplification of minute quantities of DNA, therefore the process must be set up under sterile conditions to prevent contamination. Pipette tips, tubes and water are autoclaved before use; gloves are worn at all times; reagents purchased are DNA and DNase free. If possible, initiate PCR set-up should be performed in a DNA free hood. It is important to include a negative control (no DNA) in your experiment; this will give an indication of any contamination of the PCR master mix.
Procedure:
- Pipette 4.0 µl of the appropriate cocktail directly into the bottom of a sterile tube for each reaction. The tubes should be labeled by placing a round sticker subsequent steps. on the cap to prevent smearing by oil in subsequent steps.
- Add 1.0 µl of the DNA directly into the drop of cocktail in each tube and ensure adequate mixing. Quickly spin to collect the reaction mixture to the bottom of the tube.
- Overlay each reaction with one drop of light mineral oil using a Pasteur pipette. The samples may be quick spun if necessary before placing the thermocycler.
- Place the tightly capped tubes in the temperature block and make sure each is firmly seated by pressing on the tubes individually.
- The PCR machine must now be programmed for the specific conditions desired. Each cycle in the PCR reaction involves 3 steps denaturation, annealing and primer extension) and the products are amplified by performing many cycles one after the other with the help of automated thermocycler.
- After the completion of PCR reaction remove the tubes from the temperature block.
Primer used in this study:-
| gyrA | GCTACATCCTGCTTGCCTTC | 210 bp |
| CATAGATCGCCGTGAAGAGG |
PCR conditions as follows:-
| S. No. | Treatments | Temperature | Duration |
| 1. | Predenaturation | 95 ° C | 08 min |
| 2. | Denaturation | 95 ° C | 30 sec |
| 3. | Annealing | 50 ° C | 30 sec |
| 4. | Primer extention | 72 ° C | 30 sec |
| 5. | Final extention | 72 ° C | 10 min |
Contents to be added in a PCR tube (for 20 µl):-
Primer (forward) 0.4 µl
Primer (reverse) 0.4 µl
DNTPS 0.3 µl
Mgcl2 0.3 µl
Buffer 2.0 µl
Taq polymerase 0.2 µl
DNA Sample 1.0 µl
Make-up up to 20 µl using MQ water
AGAROSE GEL ELECTROPHOREISIS
Agarose is a linear polysaccharide made up of the basic repeat unit agarobiose which comprises alternating unit of galactose and 3, 6- anhydrogalactose. For majority of DNA samples, electrophorectic separation is carried out in agarose gel. This is because most DNA molecules and their fragments that are analyzed routinely are considerably larger than protein hence most of the DNA fragments would be unable to enter polyacrylamide gel; therefore, the larger pore size of an agarose gel is required.
Procedure:
- Mix the appropriate weight of agarose with TAE buffer.
- Place the container in the microwave at full power for 4 min 30 sec, heat protecting gloves should be used when heating the agarose.
- The agarose should be dissolved, but not in boil.
- Once dully melted leave in the microwave for 2 min to cool as immediate removal can result in boiling over.
- Place at 55 C water bath to allow it to cool without setting.
- Seal the edges of a small gel-casting tray with biohazard tape.
- Add 100 µl (per 1) of ethidium bromide to the cooled agarose solution about 60 C and mix by gentle swirling.
- Insert a fine comb into the casting tray.
- Pour the agarose to a depth of about 1 cm and allow it to get solidify
- Remove the biohazard tape.
- Take 10µl of a 500µl DNA sample add 2.5 µl loading dye.
- Use 1µl of 1kb ladder (in refrigerator), 9ul water, and 2.5 µl loading dye as a marker.
- Fill tank to adjust above gel bed using TAE buffer (2L).
- Place gel in tank ensuring the gel is just submerged.
- Add the samples to the sample wells and run at 80V for about an hr or till front dye is near bottom of the gel.
- Check electrodes are bubbling so u knows that the circuit is complete.
- After the electrophoresis photograph gel under UV transillumination.
Requirements:
Agarose 0.8% agarose
8 g per liter of 10X TAE buffer 100 ml per liter
1 Kb ladder
from invitrogen use 1µl per lane
Loading dye
0.25% bromophenol blue 2.5g per 1
0.25%xylene cyanol
2.5g per 1
25% ficoll 400
250g per 1
Dissolve in 10X TAE buffer
Running buffer
0.4 M tris
48.5g per 1
0.19M glacial acetic acid 11.4 ml per 1
10 mM EDTA
3.7g per 1
pH 8
Can be made by using 100 ml TAE (10X) made upto 1 1 with water Staining dye(ethidium bromide)
100µl per 1
Ethidium bromide sol (10mg/ml)
REAL TIME -PCR (RT-PCR)
Quantitative RT-PCR is currently the “gold standard” for mRNA analysis, offering the best sensitivity, dynamic range, and reproducibility of any standard technique. In qRT-PCR, mRNA transcripts are first reverse transcribed into cDNA using oligo(dT), random oligomer, or gene-specific primers; the cDNAs Or Bacterial samples of interest are then exponentially amplified by PCR using gene-specific primers. The concentration of amplicon in the reaction is monitored with fluorophore-conjugated hybridization probes or DNA- intercalating dyes. Template quantitation is based on the number of PCR cycles required for fluorescence to reach an arbitrary threshold. Low-order multiplexing (2-5 targets) is feasible using multiple primer pairs and different-colored probes, but tends to be problematic due to formation of “primer dimer” side products and competition between assay targets. Moreover, since very small amounts of template are required, evaluation of multiple targets can usually be achieved more easily by aliquoting sample to parallel simplex qPCR reactions.
Preparation of Master mix for qPCR
| 1. | RT PCR Master mix | 5µl |
| 2. | Primer Forward | 1µl |
| 3. | Primer Reverse | 1µl |
| 4. | Bacterial diluted sample | 1µl |
| 5. | Nuclease free distilled water | 2µl |
| Total Volume | 10µl |
DAIGRAM
RESULTS AND DISCUSSION
Antibiotic resistance in bacterial isolates from poultry has become a critical issue in the realm of food safety and public health. The thesis focuses on addressing this growing concern by employing advanced molecular techniques for the detection of antibiotic resistance genes in bacterial populations from poultry samples. In initial stages of the study, 10 samples of chicken were obtained and prepared for inoculation on nutrient agar media. Subsequently, the inoculum was cultured on agar plates, leading to the observation of various colonies. This preliminary step serves as the foundation for further investigation into the bacterial species present in the chicken samples and their potential antibiotic resistance profiles. The use of qPCR (quantitative polymerase chain reaction) technique provides a powerful tool for the rapid and accurate detection of antibiotic resistance genes within bacterial isolates. By combining traditional microbiological methods with molecular biology techniques, this thesis aims to shed light on the prevalence and types of antibiotic resistance in bacteria derived from poultry sources, contributing valuable insights into the dynamics of antibiotic resistance in the poultry industry. Randomly picked colonies were subjected to further inoculated in LB broth for the recovery of enough quantity of bacteria for genome extraction. The DNA from 20 bacterial isolates were successfully extracted using phenol chloroform method as shown in figure 4.2.

Figure 4.1 :- Bacterial colony on nutrient agar media

Figure 4.2 :- Bacterial DNA extracted from different bacterial isolates for PCR
After genome extraction using phenol chloroform methods and we performed PCR for molecular identification of antibiotic resistant gene tetA and we also directly applied RTPCR on diluted chicken sample in autoclave distilled water for the rapid assessment of the tetA gene in the samples. We have molecularly analyzed genomes from the 20 Bacterial Isolates after PCR of antibiotic resistant gene tet(A) we recorded in 3 samples positive for this mutant. In this study, randomly picked colonies from chicken samples were subjected to inoculation in LB broth to recover sufficient quantities of bacteria for genome extraction. DNA extraction was successfully conducted using the phenol-chloroform method, as depicted in Figure 4.2. Subsequently, PCR was performed to molecularly identify the antibiotic-resistant gene tetA, and RT-PCR was directly applied to diluted chicken samples in autoclave-distilled water for the rapid assessment of the tetA gene presence.
The molecular analysis of genomes from the 20 bacterial isolates revealed interesting findings. After PCR amplification of the antibiotic-resistant gene tetA, it was observed that 3 out of the 20 samples tested positive for this mutant gene. This suggests the presence of antibiotic-resistant bacteria in the chicken samples, specifically showing resistance against tetracycline, as shown in Figure 4.3, Figure 4.4 illustrates the application of diluted genome samples (derived from chicken samples) in an RT-PCR plate to detect the tetA gene. This method allows for a rapid assessment of antibiotic resistance gene presence in the chicken samples, providing valuable insights into the potential antibiotic resistance profiles of the bacterial isolates.
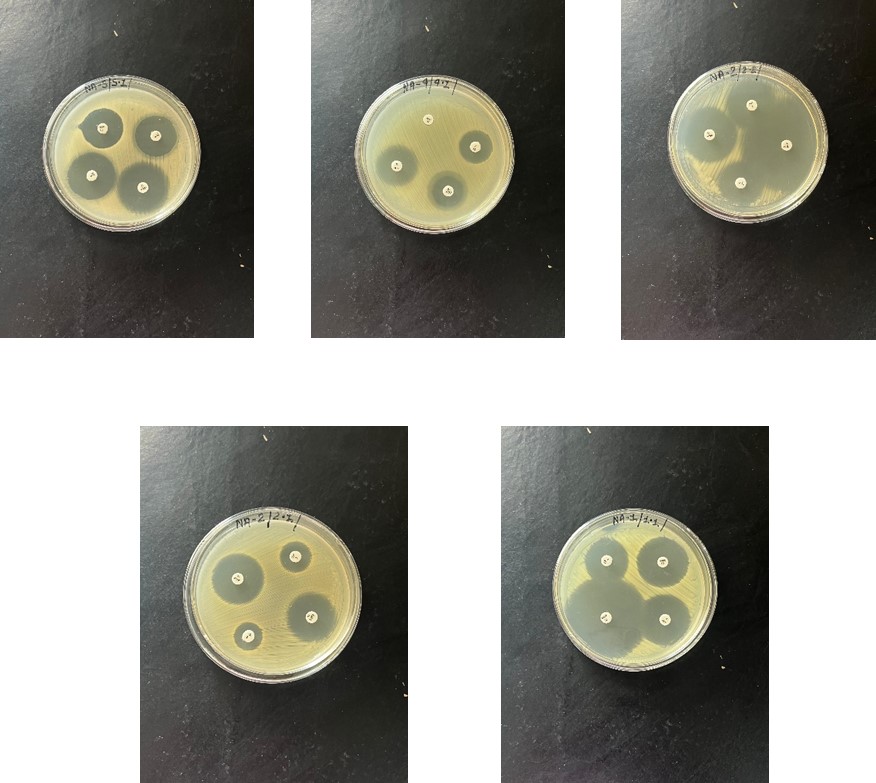
Figure 4.3 :- Antibiotic resistent bacteria showing resistance against tetracyclin.

Figure 4.4 :- Figure showing the diluted water samples (chicken samples) applied in RT-PCR plate for the detection of the tet(A) gene.
| S.No. | TetA mutant gene |
| 1 | _ |
| 2 | _ |
| 3 | _ |
| 4 | _ |
| 5 | _ |
| 6 | _ |
| 7 | _ |
| 8 | + |
| 9 | _ |
| 10 | _ |
| 11 | + |
| 12 | _ |
| 13 | _ |
| 14 | _ |
| 15 | _ |
| 16 | _ |
| 17 | + |
| 18 | _ |
| 19 | _ |
| 20 | _ |
Table 4.1: This table showing the presence on antibiotic resistant gene tetA in bacterial genome samples isolated from chicken samples.
Table 4.1 presents a summary of the results, showing the presence or absence of the antibiotic-resistant gene tetA in the bacterial samples isolated from chicken sources. Out of the 20 isolates tested, 3 were positive for the tetA mutant gene, indicating a subset of antibiotic- resistant bacterial strains within the chicken samples. These findings underscore the importance of monitoring antibiotic resistance in poultry bacterial isolates and highlight the utility of molecular techniques like PCR and RT-PCR in quickly identifying antibiotic resistance genes. Such insights are crucial for understanding the dynamics of antibiotic resistance in food-producing animals and informing strategies for mitigating the spread of antibiotic resistance in both animal and human populations.
CONCLUSION
The study successfully identified antibiotic-resistant bacterial strains in chicken samples, with 3 out of 20 isolates testing positive for the tetA gene. These findings emphasize the need for continued monitoring of antibiotic resistance in poultry and highlight the importance of utilizing molecular techniques for rapid detection. Understanding and addressing antibiotic resistance in food-producing animals is crucial for safeguarding public health and informing effective antimicrobial stewardship practices.
REFERENCE
- Bassett, E. J., Keith, M. S., Armelagos, G. J., Martin, D. L., and Villanueva, A. R. (1980). Tetracycline-labeled human bone from ancient Sudanese Nubia (A.D. 350). Science 209, 1532-1534.
- Nelson, M. L., Dinardo, A., Hochberg, J., and Armelagos, G. J. (2010).
- Brief communication: mass spectroscopic characterization of tetracycline in the skeletal remains of an ancient population from Sudanese Nubia 350-550 CE. Am. J. Phys. Anthropol. 143, 151-154.
- Kobayashi, T., Nonaka, L., Maruyama, F., and Suzuki, S. (2007). Molecular evidence for the ancient origin of the ribosomal protection protein that mediates tetracycline resistance in bacteria. J. Mol. Evol. 65, 228-235.
- Aminov, R. I., and Mackie, R. I. (2007). Evolution and ecology of antibiotic resistance genes. FEMS Microbiol. Lett. 271, 147-161.
- Hall, B. G., and Barlow, M. (2004). Evolution of the serine beta- lactamases: past, present and future. Drug Resist. Updat. 7, 111-123.
- Garau, G., Di Guilmi, A. M., and Hall, B. G. (2005). Structure-based phylogeny of the metallo-beta-lactamases. Antimicrob. Agents Chemother. 49, 2778-2784.
- Fevre, C., Jbel, M., Passet, V., Weill, F. X., Grimont, P. A., and Brisse, S. (2005). Six groups of the OXY ẞ-lactamase evolved over millions of years in Klebsiella oxytoca. Antimicrob. Agents Chemother. 49, 3453- 3462.
- Walker, D. & Fowler, T. Annual Report of the Chief Medical Officer: Volume Two, 2011: Infections and the Rise of Antimicrobial Resistance (Department of Health, 2011).
- World Economic Forum. Global Risks 2013 Eighth Edition http://www.weforum.org/reports/global-risks-2013-eighth-edition (2013).
- World Economic Forum. Global Risks http://www.weforum.org/reports/global-risks-2014- 2014 Report – report (2014).
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014 http://www.who. int/drugresistance/documents/surveillancereport/en/ (2014).
- Hampton, T. Report reveals scope of US antibiotic resistance threat. JAMA 310, 1661-1663 (2013).
- 13.Chuanchuen, R., Karkhoff-Schweizer, R. R. & Schweizer, H. P. High- level triclosan resistance in Pseudomonas aeruginosa is solely a result of efflux. Am. J. Infect. Control 31, 124-127 (2003).
- Zhu, L., Lin, J., Ma, J., Cronan, J. E. & Wang, H. Triclosan resistance of Pseudomonas aeruginosa PAO1 is due to FabV, a triclosan-resistant enoyl-acyl carrier protein reductase. Antimicrob. Agents Chemother. 54, 689-698 (2010).
- Lesher GY. Froelich EJ. Gruett MD. et al. 1.8 Naphtyridine derivatives. A new class of chemotherapeutic agents. J Med Pharmacol Chern 1962;5:1063-5.
- Wolfson JS. Hooper De. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev 1989;2:378-424.
- Holmes B. Brogden RN. Richards OM. Norfloxacin: a review of its antibacterial activity. pharmacokinetic properties Drugs 1985;30:482-513.
- Sanders WE Jr. Efficacy. safety, and potential economic benefits of oral ciprofloxacin in the treatment of infections. Rev Infect Dis 1988; 10:528- 43.
- Monk JP. Campoli-Richards OM. Ofioxacin: a review ofits antibacterial activity. pharmacokinetic properties and therapeutic use. Drugs 1987;33:346-91.
- Griineberg RN. Felmingham D. O’Hare MD. et al. The compara tive in- vitro activity of ofioxacin. J Antimicrob Chemother 1988; 22(suppl C):9- 19.
- Hirai K. Aoyama H. Hosaka M. et al. In vitro and in vivo antibacterial activity of AM-833. a new quinolone derivative. Antimicrob Agents Chemother 1986;29: 1059-66.
- Speciale A. Stefani S. Caccamo F. Nicolosi YM. Nicoletti G. The sensitivity of gram-negative and gram-positive bacteria to ofioxacin. Drugs Exp Clin Res 1987; 13:555-61.
- Machka K, Braveny I. Comparative in vitro activity of RO 23-6240 (fleroxacin), a new 4-quinolone derivative. Eur J Clin Microbiol 1987;6:482-5.
- 24.Andreasen JJ. Andersen LP. Hartzen SH. In vitro susceptibility of diarrhoea producing gram negative enteric bacteria to sulfasalazine, 5- aminosalicylic acid. sulfapyridine and four quinolones. APMIS 1988;96:568-70.
- Mitsuhashi Comparative antibacterial activity of new quinolonecarboxylic acid derivatives. Rev Infect Dis 1988; 10(suppl
- 1):S27-31.
- 26.Piccolomini R. Cellini L. Allocati N. Di Girolamo A. Selan L. Scazzocchio F. In vitro activity of pefioxacin compared with five other quinolones on nalidixic acid-resistant Proteae species. Chemioterapia 1988;7:287-91.
- Rotter M. Hirschi AM. Untersuchungen zur antibakteriellen Wirkung von Ciprofloxacin, Ofioxacin und Norfioxacin in vitro. Zentralbl Bakt Hyg [A] 1988;270:145-52.
- Mitsuhashi S. Comparative antibacterial activity of new quinolonecarboxylic acid derivatives. Rev Infect Dis 1988; 10(suppl
- I):S27-31.
- Clark LC, Seipke RF, Prieto P, Willemse J, van Wezel GP, Hutchings MI, Hoskisson PA. Mammalian cell entry genes in Streptomyces may provide clues to the evolution of bacterial virulence. Scientific reports. 2013 Jan 23;3(1):1-8.
- Klassen JL. Microbial secondary metabolites and their impacts on insect symbioses. Current opinion in insect science. 2014 Oct 1;4:15-22.
- Zasloff M. Antimicrobial peptides of multicellular organisms. nature. 2002 Jan;415(6870):389-95.
- Pratt WB, Scholar EM, Scholar EM,. The antimicrobial drugs. Oxford University Press, USA; 2000.
- Behroozian S, Svensson SL, Davies J. Kisameet clay exhibits potent antibacterial activity against the ESKAPE pathogens. MBio. 2016 Jan 26;7(1):e01842-15.
- Sutcliffe 1A. Gootz TO. Barrett IF. Biochemical characteristics and physiological significance of major DNA topoisomerases. Antimicrob Agents Chemother 1989;33:2027-33.
- Sanders Cc. Microbiology of ftuoroquinolones. In: Sanders WE Jr. Sanders CC, eds. Fluoroquinolones in the treatment of infectious diseases. Glenview. IL: Physicians and Scientists Publishing. 1990: 1-27.
- Hoshino K. Sato K. Une T. Osada Y. Inhibitory effects ofquinolones on DNA gyrase of Escherichia coli and topoisomerase II offetal calf thymus. Antimicrob Agents Chemother 1989;33: 1816-8.
- Nakamura A, Furuta T, Shirai N, Sugimoto M, Kajimura M, Soya Y, Hishida A. Determination of mutations of the 23S r-RNA gene of Helicobacter pylori by allele specific primer-polymerase chain reaction method. Journal of gastroenterology and hepatology. 2007 Jul;22(7):1057-63.
- Ladely SR, Meinersmann RJ, Englen MD, Fedorka-Cray PJ, Harrison MA. 23S r-RNA gene mutations contributing to macrolide resistance in Campylobacter jejuni and Campylobacter coli. Foodborne pathogens and disease. 2009 Feb 1;6(1):91-8.
- Spellberg B, Talbot GH, Boucher HW, Bradley JS, Gilbert D, Scheld WM, Edwards Jr J, Bartlett JG, Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Antimicrobial agents for complicated skin and skin-structure infections: justification of noninferiority margins in the absence of placebo-controlled trials. Clinical infectious diseases. 2009 Aug 1;49(3):383-91.
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. Antimicrobial-resistant pathogens associated with healthcare-
- associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infection Control & Hospital Epidemiology. 2008 Nov;29(11):996-1011.
- Brook I, Foote PA, Hausfeld JN. Increase in the frequency of recovery of meticillin-resistant Staphylococcus aureus in acute and chronic maxillary sinusitis. Journal of medical microbiology. 2008 Aug 1;57(8):1015-7.
- Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994 Apr 15,264(5157):375-82.
- Capita R, Alonso-Calleja C. Antibiotic-resistant bacteria: a challenge for the food industry. Critical reviews in food science and nutrition. 2013 Jan 1;53(1):11-48.
- Janda JM, Abbott SL. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. Journal of clinical microbiology. 2007 Sep;45(9):2761-4.
- Dauga C. Evolution of the gyrB gene and the molecular phylogeny of Enterobacteriaceae: a model molecule for molecular systematic studies. International Journal of Systematic and Evolutionary Microbiology. 2002 Mar 1;52(2):531-47.
- Tayeb LA, Lefevre M, Passet V, Diancourt L, Brisse S, Grimont PA. Comparative phylogenies of Burkholderia, Ralstonia, Comamonas, Brevundimonas and related organisms derived from rpoB, gyrB and rrs gene sequences. Research in Microbiology. 2008 Apr 1;159(3):169-77.
- Chopra I, O’Neill AJ, Miller K. The role of mutators in the emergence of antibiotic-resistant bacteria. Drug Resistance Updates. 2003 Jun 1;6(3):137-45.
- 48.Arkali, A., & Çetinkaya, B. (2020). Molecular identification and antibiotic resistance profiling of Salmonella species isolated from chickens in eastern Turkey. BMC veterinary research, 16(1), 205.
- 49.Zhao, X., Wang, J., Zhu, L., Ge, W., & Wang, J. (2017). Environmental analysis of typical antibiotic-resistant bacteria and ARGs in farmland soil chronically fertilized with chicken manure. Science of the Total Environment, 593, 10-17.
- Racewicz, P., Majewski, M., Biesiada, H., Nowaczewski, S., Wilczyński, J., Wystalska, D., & Madeja, Z. E. (2022). Prevalence and characterisation of antimicrobial resistance genes and class 1 and 2 integrons in multiresistant Escherichia coli isolated from poultry production. Scientific Reports, 12(1), 6062.
- 51.Olonitola, O. S., Fahrenfeld, N., & Pruden, A. (2015). Antibiotic resistance profiles among mesophilic aerobic bacteria in Nigerian chicken litter and associated antibiotic resistance genes. Poultry science, 94(5), 867-874.
- 52.Savin, M., Alexander, J., Bierbaum, G., Hammerl, J. A., Hembach, N., Schwartz, T., … & Kreyenschmidt, J. (2021). Antibiotic-resistant bacteria, antibiotic resistance genes, and antibiotic residues in wastewater from a poultry slaughterhouse after conventional and treatments. Scientific Reports, 11(1), 16622. advanced
- 53.Liu, Y., Dyall-Smith, M., Marenda, M., Hu, H. W., Browning, G., & Billman-Jacobe, H. (2020). Antibiotic resistance genes in antibiotic-free chicken farms. Antibiotics, 9(3), 120.
- 54.McNeece, G., Naughton, V., Woodward, M. J., Dooley, J. S., & Naughton, P. J. (2014). Array based detection of antibiotic resistance genes in Gram negative bacteria isolated from retail poultry meat in the UK and Ireland. International journal of food microbiology, 179, 24-32.
- Thibodeau, A., Quessy, S., Guévremont, E., Houde, A., Topp, E., Diarra, M. S., & Letellier, A. (2008). Antibiotic resistance in Escherichia coli and Enterococcus spp. isolates from commercial broiler chickens receiving growth-promoting doses of bacitracin or virginiamycin. Canadian Journal of Veterinary Research, 72(2), 129.
- 56.Faldynova, M., Videnska, P., Havlickova, H., Sisak, F., Juricova, H., Babak, V., … & Rychlik, I. (2013). Prevalence of antibiotic resistance genes in faecal samples from cattle, pigs and poultry. Veterinarni Medicina, 58(6).
AUTHOR INFORMATION
Corresponding Author
Manisha Shukla
Department of Biotechnology, Pandit S.N. Shukla University, Shahdol, Madhya Pradesh, India 484001
Email: mshukla@bt.iitr.ac.in
Phone: +91- 8839096157
Authors
Anjali Tomar
Department of Biotechnology, Pandit S.N. Shukla University, Shahdol, Madhya Pradesh, India 484001
Naeem Akhtar
Department of Biotechnology, Pandit S.N. Shukla University, Shahdol, Madhya Pradesh, India 484001
Rohit Kumar Vishwakarma
Department of Biotechnology, Pandit S.N. Shukla University, Shahdol, Madhya Pradesh, India 484001
