Gallbladder Cancer: A Comprehensive Clinical Data Hub
Gallbladder cancer (GBC) is a challenging malignancy with a generally poor prognosis, often diagnosed at advanced stages. Significant efforts are underway globally to improve patient outcomes through innovative treatment strategies, from neoadjuvant therapies to targeted agents and immunotherapies. Understanding the landscape of ongoing clinical trials and the molecular underpinnings of GBC is crucial for researchers, clinicians, and patients alike.
This Gallbladder Cancer Clinical Data Hub, a curated collection of vital information on current and historical clinical trials for GBC, along with an overview of key molecular alterations driving the disease. This data empowers deeper insights into treatment efficacy, emerging therapeutic targets, and patient characteristics that influence outcomes.
Table. Ongoing clinical trials for the evaluation of neoadjuvant therapy in locally advanced gallbladder cancer. BTC: biliary tract cancer. GBC: gallbladder cancer. OS: overall survival. ORR: overall response rate. RCT: radiochemotherapy. RT: radiotherapy.

| NCT Number | Study Phase | Condition | Study Size | Treatment Agent | Primary End Point | Institution | Completition |
| NCT03673072 | III | Incidental GBC and BTC | 300 | Gemcitabine + cisplatin perioperative vs. adjuvant | OS | Krankenhaus Nordwest, Germany | Nov-24 |
| NCT02867865 | II/III | GBC | 314 | Gemcitabine + cisplatin alone vs. RT + gemcitabine + cisplatin | OS | Tata Memorial Hospital, India | Aug-22 |
| NCT04308174 | II | BTC | 45 | Gemcitabine + cisplatin vs. gemcitabine + cisplatin + durvalumab | R0 resection rate | Asan Medical Center, Korea | Dec-23 |
| NCT04559139 | II/III | GBC | 186 | Gemcitabine + cisplatin perioperative vs. adjuvant | OS | Emory University, Winship Cancer Institute, United States | Sep-23 |
| NCT04480190 | I | BTC | 12 | Gemcitabine + cisplatin followed by RCT (5FU + RT) | Therapy completion | University of Cincinnati Medical Center, United States | Feb-29 |
Table 2. Clinical trials for the palliative cytostatic chemotherapy of BTC specifically including GBC patients.
| NCTN | Phase | Condition | Study Size | Substance | Results | Reference | |
| NCT00660140 | II | BTC + GBC | 49 | gemcitabine + carboplatin | PFS 7.8 months, OS 10.6 months | [77] | |
| Not applicable | II | GBC | 20 | gemcitabine + carboplatin | ORR 36.7%, PFS 33.8 weeks | [78] | |
| NCT00009893 | II | BTC + GBC | 42 | gemcitabine + 5FU + leucovorin | PFS 4.6 months, OS 9.7 months | [79] | |
| NCT00003276 | II | BTC + GBC | 39 | irinotecan | ORR 8% | [80] | |
| NCT00033540 | II | BTC + GBC | 57 | gemcitabine + capecitabin | ORR 25%, OS 7 months | [81] | |
| NCT00059865 | II | BTC + GBC | 63 | gemcitabine + pemetrexed | No benefit of combined regimen compared to gemcitabine | [82] | |
| NCT00075504 | II | BTC + GBC | 33 | triapine + gemcitabine | ORR 9%; no benefit with triapine | [83] | |
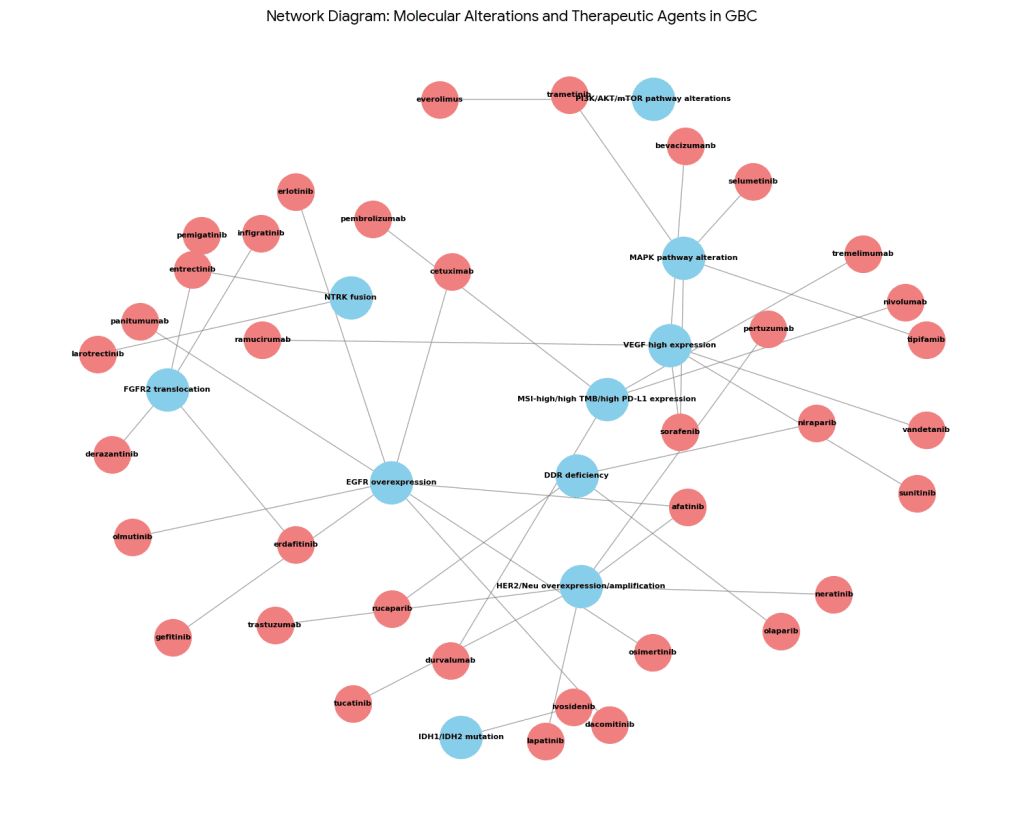
Table 3. Overview of targetable molecular alterations in GBC.
| Molecular Alteration | Frequency | Therapeutic Agents | |
| HER2/Neu overexpression/amplification | 9.8–27.3% | trastuzumab, lapatinib, neratinib, pertuzumab, afatinib, tucatinib | |
| VEGF high expression | 48% | bevacizumanb, sorafenib, sunitinib, ramucirumab, vandetanib | |
| EGFR overexpression | 44–77% | erlotinib, cetuximab, panitumumab, gefitinib, afatinib, dacomitinib, osimertinib, olmutinib | |
| MAPK pathway alteration | Up to 45% | trametinib, selumetinib, sorafenib, tipifamib | |
| PI3K/AKT/mTOR pathway alterations | 10% | everolimus | |
| MSI-high/high TMB/high PD-L1 expression | 12% | pembrolizumab, nivolumab, durvalumab, tremelimumab | |
| DDR deficiency | Up to 27% | olaparib, niraparib, rucaparib | |
| IDH1/IDH2 mutation | 2% | ivosidenib | |
| NTRK fusion | 4% | entrectinib, larotrectinib | |
| FGFR2 translocation | 3% | pemigatinib, infigratinib, derazantinib, erdafitinib | |
Sturm, N.; Schuhbaur, J.S.; Hüttner, F.; Perkhofer, L.; Ettrich, T.J. Gallbladder Cancer: Current Multimodality Treatment Concepts and Future Directions. Cancers 2022, 14, 5580. https://doi.org/10.3390/cancers14225580
Therapeutic outcomes and prognostic factors in unresectable gallbladder cancer treated with gemcitabine plus cisplatin
Table : Baseline characteristics of all patients
| Variables | N = 173 |
| Age (range) | 63.8 (25.0–84.0) |
| Sex (female / male) | 86 (49.7%) / 87 (50.3%) |
| ECOG (0 / 1 / 2) | 36 (20.8%) / 126 (72.8%) / 11 (6.4%) |
| BMI | 23.0 ± 2.8 |
| Charlson comorbidity index | 8.0 ± 1.4 |
| Stage (IIIB / IVA / IVB) | 1 (0.6%) / 8 (4.6%) / 164 (94.8%) |
| Invasion | |
| Liver | 47 (27.2%) |
| Extrahepatic bile duct | 26 (15.0%) |
| Intestine | 20 (11.6%) |
| Peritoneum | 2 (1.2%) |
| Hepatic artery | 15 (8.7%) |
| Main portal vein | 11 (6.4%) |
| Metastasis | |
| Liver | 74 (42.8%) |
| Peritoneum | 46 (26.6%) |
| Lung | 29 (16.8%) |
| Bone or muscular system | 14 (8.1%) |
| Distant lymph node | 102 (59.0%) |
| Previous history | |
| Biliary drainage | 49 (28.3%) |
| Curative surgery | 33 (19.1%) |
| Palliative chemotherapy | 12 (8.5%) |
| Baseline laboratory findings | |
| WBC (cells/μL) | 6540.0 ± 5192.4 |
| CRP (mg/dL) | 3.5 ± 4.0 |
| eGFR (mL/min/1.73m2) | 92.8 ± 23.1 |
| AST (IU/L) | 40.3 ± 40.3 |
| ALT (IU/L) | 44.2 ± 50.8 |
| ALP (IU/L) | 183.7 ± 174.9 |
| Bilirubin, total (mg/dL) | 1.5 ± 2.4 |
| CEA (ng/mL) | 68.6 ± 247.7 |
| CA 19–9 (U/mL) | 2676.9 ± 6783.1 |
| NLR | 4.2 ± 3.4 |
| PLR | 189.0 ± 95.0 |
Table 2 Treatment data and efficacy of GEMCIS in unresectable gallbladder cancer
| Variables | N = 173 |
| Treatment duration, months | 3.8 ± 3.9 |
| Total cycle | 5.3 ± 4.4 |
| OS, months (95% CI) | 8.1 (7.1–10.2) |
| PFS, months (95% CI) | 5.6 (4.5–6.8) |
| Best response | |
| CR | 2 (1.2%) |
| PR | 26 (15.0%) |
| SD | 75 (43.4%) |
| PD | 48 (27.7%) |
| NE | 22 (12.7%) |
| ORR (CR + PR) | 28 (16.2%) |
| DCR (CR + PR + SD) | 103 (59.5%) |
| Number of cycles | |
| 1 | 38 (22.0%) |
| 2 | 31 (18.0%) |
| 3 | 8 (4.7%) |
| 4 | 16 (9.3%) |
| 5 | 10 (5.8%) |
| 6 | 18 (10.5%) |
| 7 | 6 (3.5%) |
| 8 | 14 (8.1%) |
| 9 | 2 (1.2%) |
| ≥ 10 | 30 (17.4%) |
Table Univariable analysis of possible factors affecting overall survival
| Number of patients (%) | Median OS, months (95% CI) | HR (95% CI) | P value | ||
| Age | |||||
| < 65 | 90 (52.0%) | 10.1 (7.6–11.4) | 1 | ||
| ≥ 65 | 83 (48.0%) | 7.2 (6.0–10.0) | 1.28 (0.92–1.78) | 0.138 | |
| Sex (female/ male) | |||||
| Female | 86 (49.7%) | 10.1 (7.8–12) | 1 | ||
| Male | 87 (50.3%) | 7.1 (5.5–10.1) | 1.22 (0.88–1.69) | 0.234 | |
| ECOG | |||||
| 0–1 | 164 (94.8%) | 8.1 (7.1–10.3) | 1 | ||
| 2 | 9 (5.2%) | 7.5 (2.8-NE) | 1.86 (0.94–3.68) | 0.074 | |
| BMI | |||||
| > 25 | 136 (78.6%) | 7.8 (6.6–9.7) | 1 | ||
| ≥ 25 | 37 (21.4%) | 11.9 (7.1–15.1) | 0.79 (0.53–1.18) | 0.255 | |
| Stage | |||||
| IIIB/ IVA | 9 (5.2%) | 7.2 (2.1-NE) | 1 | ||
| IVB | 164 (94.8%) | 8.1 (7.1–10.3) | 0.68 (0.35–1.35) | 0.272 | |
| Charlson comorbidity index | |||||
| < 9 | 110 (63.6%) | 9.7 (7.8–11.0) | 1 | ||
| ≥ 9 | 63 (36.4%) | 6.4 (4.4–10.2) | 1.23 (0.87–1.72) | 0.237 | |
| Local invasion | |||||
| Liver | 47 (27.2%) | 7.7 (6.6–11.4) | 1.47 (1.02–2.12) | 0.04 | |
| Extrahepatic bile duct | 26 (15.0%) | 6.5 (5.0–11.9) | 1.06 (0.67–1.67) | 0.812 | |
| Intestine | 20 (11.6%) | 8.6 (5.0-NE) | 0.79 (0.47–1.34) | 0.386 | |
| Peritoneum | 2 (1.2%) | 10.5 (2.3-NE) | 0.94 (0.23–3.82) | 0.933 | |
| Hepatic artery | 15 (8.7%) | 6.4 (5.0-NE) | 1.12 (0.63–1.98) | 0.699 | |
| Portal vein | 11 (6.4%) | 5.9 (2.3-NE) | 1.17 (0.6–2.31) | 0.643 | |
| Metastasis site | |||||
| Liver | 74 (42.8%) | 6.2 (5.3–10.0) | 1.72 (1.23–2.41) | 0.002 | |
| Peritoneum | 46 (26.6%) | 6.5 (4.2–10.1) | 1.25 (0.87–1.82) | 0.229 | |
| Lung | 29 (16.8%) | 7.2 (5.4–15.6) | 0.85 (0.54–1.34) | 0.487 | |
| Bone or muscular system | 14 (8.1%) | 5.1 (3.3–13.1) | 2.03 (1.14–3.61) | 0.016 | |
| Distant lymph node | 102 (59.0%) | 8.3 (6.5–10.9) | 1.10 (0.79–1.54) | 0.56 | |
| Total bilirubin | |||||
| ≤ 1.5 X ULN | 130 (75.1%) | 9.7 (7.8–11.0) | 1 | ||
| > 1.5 X ULN | 43 (24.9%) | 5.3 (3.7–9.5) | 1.77 (1.21–2.58) | 0.003 | |
| Transaminase | |||||
| ≤ 1.5 X ULN | 120 (69.4%) | 9.7 (7.8–11.5) | 1 | ||
| > 1.5 X ULN | 53 (30.6%) | 6.2 (4.5–9.7) | 1.53 (1.07–2.18) | 0.019 | Outcome |
| NLR | PR: 3.8%, mPFS: 1.64 months, mOS: 6.4 months | ||||
| ≤ 3 | 75 (43.4%) | 12.4 (10.2–14.6) | 1 | PR: 37%, mPFS: 4.2 months, mOS: 15.4 months | |
| > 3 | 98 (56.6%) | 6.2 (4.8–7.8) | 2.34 (1.66–3.29) | < 0.001 | PR: 3%, mPFS: 1.4 months, mOS: 5.2 months |
| PLR | PR: 22%, DCR: 60%, mPFS: 4 months, mOS: 14.2 months | ||||
| < 190 | 102 (59.0%) | 10.3 (9.4–13.7) | 1 | PR: 13%, SD: 17%, mPFS: 1.8 months, mOS: 6.2 months | |
| ≥ 190 | 71 (41.0%) | 6.6 (5.6–8.1) | 1.77 (1.27–2.47) | 0.001 | PR: 5.8%, mPFS: 2 months, mOS: 7.4 months |
| CEA, ng/mL | PR: 48%, mPFS: 1.5 months, mOS: 8.1 months | ||||
| < 5 | 103 (59.5%) | 10.3 (7.8–13.1) | 1 | PR: 11%, mPFS: 1.6 months, mOS: 10.1 months | |
| ≥ 5 | 70 (40.5%) | 6.5 (5.0–8.1) | 1.87 (1.33–2.63) | < 0.001 | PR: 12.5%, mPFS: 3.4 months, mOS: 6 months |
| CA 19–9, U/mL | ORR: 20%, mOS: 12.7 months | ||||
| < 500 | 114 (65.9%) | 10.7 (9.0–13.3) | 1 | ||
| ≥ 500 | 59 (34.1%) | 5.5 (4.1–7.6) | 2.28 (1.61–3.23) | < 0.001 | |
https://bmccancer.biomedcentral.com/articles/10.1186/s12885-018-5211-y#Tab2
Table : Ongoing clinical trials evaluating gallbladder cancer (GBC)
| Drug investigated | Molecular target | Target population | Phase | Clinical trail ID | Locations |
| Sorafenib | Multitargeted TKI | GBC | 2 | NCT00238212 | USA |
| Sorafenib | Multitargeted TKI | Extrahepatic bile duct cancer, GBC | 2 | NCT00919061 | USA |
| Sorafenib | Multitargeted TKI | BTC | 1 and 2 | NCT00955721 | USA |
| Sorafenib | Multitargeted TKI | GBC | 3 | NCT01053390 | China |
| KBP-5209 | Multitargeted TKI | Solid tumors | 1 | NCT02442414 | USA |
| Erlotinib | EGFR | Solid tumors | 1 | NCT00397384 | USA |
| Bevacizumab | EGFR, VEGFR | Upper gastrointestinal cancers | 2 | NCT00350753 | Denmark |
| Bevacizumab | EGFR, VEGFR | BTC | 2 | NCT00356889 | USA |
| Bevacizumab | EGFR, VEGFR | BTC | 2 | NCT00361231 | USA |
| Bevacizumab | EGFR, VEGFR | BTC | 2 | NCT01007552 | USA |
| Afatinib | EGFR, HER2 | GBC | 2 | NCT04183712 | China |
| Apatinib | EGFR, HER2 | GBC | 2 | NCT03702491 | China |
| Lapatinib | HER2 | BTC | 2 | NCT00101036 | USA |
| Lapatinib | HER2 | BTC | 2 | NCT00107536 | USA |
| Trastuzumab, R115777 | HER2 | Solid tumors | 1 | NCT00005842 | USA |
| Trastuzumab | HER2 | Advanced or metastatic GBC | 2 | NCT00478140 | USA |
| Trastuzumab, IL-12 | HER2, IL-12 | Solid tumors | 1 | NCT00004074 | USA |
| IL-2 | HER2 | Solid tumors | 1 | NCT02662348 | China |
| Cediranib | VEGFR | BTC | 2 | NCT01229111 | USA |
| Ramucirumab | VEGFR2 | BTC | 2 | NCT02520141 | USA |
| Ramucirumab, merestinib | VEGFR2, c-MET | BTC | 2 | NCT02711553 | USA |
| Pazopanib | VEGFR1, VEGFR2, VEGFR3, PDGFRβ, c-Kit, FGFR1, c-Fms | BTC | 2 | NCT01855724 | Greece |
| Vandetanib | VEGFR2–3, EGFR, RET | Advanced BTC | 2 | NCT00753675 | Italy |
| Regorafenib | VEGFR1–3, PDGFRβ, KIT, RET Raf-1 | BTC | 2 | NCT02115542 | USA |
| Regorafenib | VEGFR1–3, PDGFRβ, KIT, RET Raf-2 | BTC | 2 | NCT02053376 | USA |
| Panitumumab | Kras, BRAF | BTC | 2 | NCT01308840 | USA |
| Selumetinib | MEK | BTC | 1 | NCT01242605 | United Kingdom |
| Selumetinib | MEK | BTC | 2 | NCT02151084 | Canada |
| Atezolizumab | MEK | BTC | 2 | NCT03201458 | USA |
| Trametinib | MEK | BTC or GBC | 2 | NCT02042443 | USA |
| Trametinib | MEK | BTC | 2 | NCT01943864 | Japan |
| ARRY-438162 | MEK | Solid tumors | 1 | NCT00959127 | USA |
| GSK1120212 | MEK | Solid tumors | 1 | NCT01324258 | Japan |
| MEK162 | MEK | BTC | 1 | NCT02105350 | USA |
| MEK162 | MEK | BTC | 1 and 2 | NCT01828034 | USA |
| MEK162 | MEK | BTC | 1 and 2 | NCT02773459 | Korea |
| Everolimus | mTOR | Solid tumors | 1 | NCT00949949 | USA |
| Nivolumab | PD-1 | BTC | 2 | NCT02829918 | USA |
| Nivolumab | PD-1 | BTC | 2 | NCT03101566 | USA |
| Pembrolizumab | PD-1 | BTC | 2 | NCT03260712 | Spain |
| Pembrolizumab | PD-1 | BTC | 2 | NCT03111732 | USA |
| Pembrolizumab | PD-1 | BTC | 3 | NCT04003636 | USA |
| M7824 | PD-1 | BTC | 2 | NCT03833661 | USA |
| Toripalimab + lenvatinib | PD-1 | BTC | 2 | NCT04211168 | China |
| Nivolumab, ipilimumab | PD-1, CTLA-4 | Solid tumors | 2 | NCT02834013 | USA |
| STI-3031 | PD-L1 | BTC | 2 | NCT03999658 | USA |
| Avelumab | PD-L1 | Solid tumors | 1 and 2 | NCT04068194 | USA |
| Durvalumab | PD-L1 | BTC | 2 | NCT04308174 | Korea |
| Durvalumab/tremelimumab | PD-L1, CTLA-4 | BTC | 2 | NCT03473574 | Germany |
| Intrafusp alfa | PD-L1, TGF-β | BTC | 2 and 3 | NCT04066491 | USA |
| Selumetinib | AKT | BTC | 2 | NCT01859182 | USA |
| MK-2206 | AKT | BTC | 2 | NCT01425879 | USA |
| IL-12 | IL-12 | Solid tumors | 1 | NCT00003046 | USA |
| IL-12 | IL-12 | Solid tumors | 1 | NCT00003439 | USA |
| Guadecitabine | DNMT | Advanced liver, pancreatic, BTC, GBC | 1 | NCT03257761 | USA |
| CEA RNA-pulsed DC cancer vaccine | CEA | Solid tumors | 1 | NCT00004604 | USA |
| EphB4-HSA fusion protein | EphB4, HSA | Solid tumors | 1 | NCT02495896 | USA |
| ADH-1 | N-cadherin | Solid tumors | 1 | NCT01825603 | USA |
| CPI-613 | PDH, α-KGDH | BTC | 1 and 2 | NCT04203160 | USA |
| Glivec | ABL, KIT, PDGFR | BTC | 2 | NCT01153750 | Germany |
| DKN-01 | DKK1 | BTC | 1 | NCT02375880 | USA |
| PSMA/PRAME | T cells | Solid tumors | 1 | NCT00423254 | USA |
| Merestinib | MET | Solid tumors | 1 | NCT03027284 | Japan |
| FT-2102 | IDH1 | Solid tumors | 1 and 2 | NCT03684811 | USA |
| Entinostat | HDAC | Solid tumors | 1 | NCT00020579 | USA |
| CGX1321 | PORCN | Solid tumors | 1 | NCT03507998 | China |
| Ceralasertib | PARP | Solid tumors | 2 | NCT03878095 | USA |
Table : PD-L1/PD-1 studies with reported outcome in GBC
| Clinical trail number | Study phase | Treatment agent | Checkpoint target | Number of patients | Outcome |
| NCT02443324 | I | Pembrolizumab + ramucirumab | PD-1 + VEGFR | 26 | PR: 3.8%, mPFS: 1.64 months, mOS: 6.4 months |
| JapicCTI-153098 | I | Nivolumab | PD-1 | 34 (33% GBC) | PR: 37%, mPFS: 4.2 months, mOS: 15.4 months |
| Nivolumab with chemotherapy | 30 (33% GBC patients) | PR: 3%, mPFS: 1.4 months, mOS: 5.2 months | |||
| NCT02829918 | II | Nivolumab | PD-1 | 54 (26% GBC) | PR: 22%, DCR: 60%, mPFS: 4 months, mOS: 14.2 months |
| NCT02054806 | I | Pembrolizumab | PD-1 | 24 (membranous PD-L1 ≥1%) | PR: 13%, SD: 17%, mPFS: 1.8 months, mOS: 6.2 months |
| NCT02628067 | II | Pembrolizumab | PD-1 | 104 | PR: 5.8%, mPFS: 2 months, mOS: 7.4 months |
| NCT01938612 | II | Duvalumab with/without tremelimumab | PD-L1 | 42 (45% GBC) | PR: 48%, mPFS: 1.5 months, mOS: 8.1 months |
| PD-L1 + CTLA-4 | 65 (25% GBC) | PR: 11%, mPFS: 1.6 months, mOS: 10.1 months | |||
| NCT01853618 | I | Tremelimumab + RFA | PD-L1 + CTLA-4 | 20 (10% GBC) | PR: 12.5%, mPFS: 3.4 months, mOS: 6 months |
| NCT02699515 | I | M7824 | PD-L + TGF-β | 30 (40% GBC) | ORR: 20%, mOS: 12.7 months |
https://www.nature.com/articles/s41392-020-00324-2#Tab2
Table dataset summarizing the targeted therapy drugs for gallbladder cancer
| Target | Drug Name(s) | Brand Name(s) | Mutation/Gene Targeted | Line of Use | Route | Common Side Effects | Serious Side Effects |
| FGFR2 | Pemigatinib, Futibatinib | Pemazyre, Lytgobi | FGFR2 fusion/mutation | After ≥1 prior chemo, unresectable/metastatic | Oral (once daily) | Kidney issues, hair loss, diarrhea, nail issues, dry mouth/eyes, fatigue, abdominal pain, taste changes, skin issues | None listed as serious; standard monitoring for mineral level changes recommended |
| IDH1 | Ivosidenib | Tibsovo | IDH1 mutation | After prior treatment, advanced/metastatic | Oral (once daily) | Fatigue, nausea, vomiting, belly pain, cough, diarrhea, anemia, rash | Heart rhythm changes, pneumonia, jaundice |
| NTRK | Larotrectinib, Entrectinib | Vitrakvi, Rozlytrek | NTRK gene fusion | Advanced/metastatic, no prior systemic therapy | Oral (once/twice) | Liver enzyme rise, low WBC/RBC, tiredness, joint pain, GI symptoms | Mood/mental changes, heart/liver damage, vision issues, fetal harm |
| RET | Selpercatinib, Pralsetinib | Retevmo, Gavreto | RET gene rearrangement | Advanced/metastatic | Oral (once/twice) | Dry mouth, high BP, fatigue, rash, swelling, low blood counts, GI issues | Liver/lung damage, allergic reactions, heart rhythm issues, bleeding, wound healing delay |
| BRAF | Dabrafenib + Trametinib | Tafinlar + Mekinist | BRAF V600E mutation | After prior treatment, advanced/metastatic | Oral (daily) | Skin issues, rash, photosensitivity, headache, joint pain, GI symptoms | Heart, liver, lung damage, allergic reactions, eye/skin problems, increased sugar, skin cancer |
| KRAS | Adagrasib | Krazati | KRAS G12C mutation | After ≥1 prior treatment, advanced/metastatic | Oral (twice daily) | Diarrhea, nausea, fatigue, cough, low WBC/RBC, muscle pain | Lung damage (ILD), kidney/liver injury, QT prolongation (heart rhythm) |
Yes, here’s a compiled dataset-style summary of the information you provided on Hepatic Artery Infusion (HAI) and systemic chemotherapy for gallbladder cancer:
💉 Hepatic Artery Infusion (HAI)
| Aspect | Details |
| Purpose | Delivers chemotherapy directly into the hepatic artery to target liver and gallbladder tumors |
| Rationale | The hepatic artery feeds most gallbladder tumors; direct infusion increases local drug concentration |
| Benefits | – Higher drug delivery to tumor- Reduced systemic exposure- Potentially fewer side effects |
| Limitations | – Requires surgical placement of a catheter- Many patients may not be eligible due to poor health |
| Current Status | Investigational in gallbladder cancer; not standard of care yet |
Systemic Chemotherapy for Gallbladder Cancer
| Chemotherapy Drug | Brand Name | Drug Type | Usage |
| Gemcitabine | Gemzar® | Antimetabolite | Often used alone or in combo with cisplatin |
| Cisplatin | Platinol® | Platinum-based agent | Commonly combined with gemcitabine |
| 5-Fluorouracil (5-FU) | — | Antimetabolite | Used alone or with radiation therapy |
| Capecitabine | Xeloda® | Oral prodrug of 5-FU | Used with radiation or as oral chemo |
| Oxaliplatin | Eloxatin® | Platinum-based agent | Alternative to cisplatin in some regimens |
| Nab-paclitaxel | Abraxane® | Taxane (microtubule inhibitor) | Used in advanced or metastatic settings |
Combination Therapy
- Gemcitabine + Cisplatin: Standard first-line treatment; shown to improve survival over gemcitabine alone
- Chemo + Radiation: Often includes 5-FU or capecitabine for radiosensitization
Common Chemotherapy Side Effects
| Affected System | Common Side Effects |
| Bone Marrow | Low blood cell counts (anemia, neutropenia, thrombocytopenia) |
| Gastrointestinal (GI) | Nausea, vomiting, diarrhea, mouth sores, loss of appetite |
| Hair Follicles | Hair loss (alopecia) |
| General | Fatigue, weakness, increased risk of infection |
Note: Side effects vary by drug, dose, and patient health. Some are manageable with supportive care or dose adjustments.
Table : Genetic Alterations and Targeted Therapies in Cholangiocarcinoma and Gallbladder Carcinoma
| Cancer Type | Genetic Alteration | Frequency (%) | Targeted Therapies |
| Intrahepatic Cholangiocarcinoma (iCCA) | TP53 mutation | 30 | |
| IDH mutations | 20 | Ivosidenib | |
| FGFR2 fusions/rearrangements | 20 | Pemigatinib, futibatinib, infigratinib, lirafugratinib | |
| CDKN2A/B loss | 15 | ||
| KRAS mutations | 15 | ||
| ARID1A mutation | 15 | ||
| BAP1 mutations | 13 | ||
| NTRK fusions | 4 | Larotrectinib, entrectinib | |
| BRAF mutation | 3 | Dabrafenib-trametinib | |
| Extrahepatic Cholangiocarcinoma (eCCA) | TP53 mutation | 40 | |
| (perihilar [pCCA] + distal [dCCA]) | KRAS mutations | 30 | |
| SMAD4 mutation | 21 | ||
| CDKN2A/B loss | 17 | ||
| HER2 amplification/overexpression | 15 | Trastuzumab-pertuzumab, trastuzumab-deruxtecan, zanidatamab | |
| ARID1A mutation | 12 | ||
| PIK3CA mutation | 10 | ||
| NTRK fusion | 4 | Larotrectinib, entrectinib | |
| BRAF mutation | 3 | Dabrafenib-trametinib | |
| IDH mutation | 3 | Ivosidenib | |
| Gallbladder Carcinoma (GBC) | TP53 mutation | 53 | |
| HER2 amplification/overexpression | 20 | Trastuzumab-pertuzumab, trastuzumab-deruxtecan, zanidatamab | |
| ARID1A mutation | 13 | ||
| KRAS mutations | 10 | ||
| CDKN2A/B loss | 10 | ||
| PIK3CA mutation | 10 | ||
| NTRK fusions | 4 | Larotrectinib, entrectinib | |
| BRAF mutation | 3 | Dabrafenib-trametinib | |
| FGFR2 fusions/rearrangements | 3 | Pemigatinib, futibatinib, infigratinib, lirafugratinib |
First-Line Chemotherapy Clinical Trials Table
| Trial (Phase, Year) | Treatment | Study Population | Primary Endpoint | Secondary Endpoints |
| ABC-02 (Phase III, 2010) | Gemcitabine + Cisplatin vs. Gemcitabine | 410 chemo-naive patients with unresectable, recurrent, or metastatic BTC (1:1) | OS: 11.7 vs 8.1 mo, HR 0.64, P < 0.001 | PFS: 8.0 vs 5.0 mo, HR 0.63; DCR: 81.4% vs 71.8%, P = 0.049; AEs similar |
| BT22 (Phase II, 2010) | Gemcitabine + Cisplatin vs. Gemcitabine | 84 chemo-naive patients with unresectable or metastatic BTC (1:1) | OS: 11.2 vs 7.7 mo, HR 0.69, P = 0.139 | PFS: 5.8 vs 3.7 mo; 6-mo PFS: 47.4% vs 27.7%; 1-yr survival: 39% vs 31% |
| TOPAZ-1 (Phase III, 2022) | GemCis + Durvalumab vs. GemCis + Placebo | 685 chemo-naive patients with unresectable/metastatic BTC (1:1) | OS: 12.8 vs 11.5 mo, HR 0.80, P = 0.021 | PFS: 7.2 vs 5.7 mo, HR 0.75; ORR: 26.7% vs 18.7%; DOR: 6.4 vs 6.2 mo; 1-yr/2-yr OS: 54.1%/24.9% vs 48%/10.4% |
| Keynote 966 (Phase III, 2023) | GemCis + Pembrolizumab vs. GemCis + Placebo | 1069 chemo-naive patients with unresectable/metastatic BTC (1:1) | OS: 12.7 vs 10.9 mo, HR 0.83, P = 0.0034 | Grade 3–4 AEs similar between arms |
| SWOG 1815 (Phase III) | GemCis + Nab-paclitaxel vs. GemCis | 441 chemo-naive patients with unresectable/metastatic BTC (2:1) | OS: 14.0 vs 12.7 mo, HR 0.93, P = 0.58 | ORR: 34% vs 25%, P = 0.11; PFS: 8.2 vs 6.4 mo; ≥Grade 3 hematologic AEs: 60% vs 45%, P = 0.003 |
| PRODIGE 38 (Phase II–III, 2021) | mFOLFIRINOX vs. GemCis | 191 patients with unresectable/metastatic BTC (1:1) | 6-mo PFS rate: 44.6% vs 47.3% | PFS: 6.2 vs 7.4 mo; ORR: 25% vs 19.4%; OS: 11.7 vs 13.8 mo |
| FUGA-BT (Phase II, 2019) | Gem + S-1 vs. GemCis | 354 chemo-naive patients with unresectable or recurrent BTC (1:1) | OS: 15.1 vs 13.4 mo, HR 0.945; P for non-inferiority = 0.0046 | PFS: 6.8 vs 5.8 mo; ORR: 29.8% vs 32.4%; AEs: 29.9% vs 35.1% |
| KHBO1401-MITSUBA (Phase III, 2022) | GemCis + S-1 vs. GemCis | 246 chemo-naive patients with unresectable or recurrent BTC (1:1) | OS: 13.5 vs 12.6 mo, HR 0.791, P = 0.046 | PFS: 7.4 vs 5.5 mo, HR 0.748, P = 0.015; ORR: 51.5% vs 15%, P < 0.001 |
| NIFE (Phase II, 2021) | Nal-IRI + 5-FU + LV vs. GemCis | 93 patients with advanced cholangiocarcinoma (1:1) | 4-mo PFS rate: 51% vs 59.5% | iCCA PFS: 41.2% vs 71.9%; eCCA PFS: 73.3% vs 20.0%; OS: 15.9 vs 13.63 mo; eCCA OS: 18.23 vs 6.34 mo |
Table : Selected Trials of Second-Line Chemotherapy in Advanced BTC (“All Comers”)
| Trial (Phase, Year) | Treatment | Study Population | Primary Endpoint | Secondary Endpoints |
| ABC-06 (Phase III, 2021) | FOLFOX + Active Symptom Control (ASC) vs. ASC | 162 patients (1:1) with locally advanced or metastatic BTC; progressed after GemCis | OS: 6.2 vs. 5.3 months, HR 0.69 (95% CI: 0.50–0.97), P = 0.031 | 12-mo OS rate: 25.9% vs. 11.4%; PFS: 4.0 months (FOLFOX); ORR: 5%; DCR: 33% |
| NIFTY (Phase IIb, 2021, updated 2023) | Nal-IRI + 5-FU + LV vs. 5-FU + LV | 174 patients (1:1) with metastatic BTC; progressed after first-line GemCis | PFS: 4.2 vs. 1.7 months, HR 0.61 (95% CI: 0.44–0.86), P = 0.004 | OS and ORR data not provided in update |
| NCT03464968 (Phase II, 2021) | mFOLFIRI vs. mFOLFOX | 118 patients (1:1) with locally advanced or metastatic BTC; progressed after GemCis | OS: 5.7 (mFOLFIRI) vs. 6.3 months (mFOLFOX), P = 0.677 | 6-mo OS: 44.1% vs. 54.1%; PFS: 2.1 vs. 2.8 months; ORR: 4.0% vs. 5.9%; DCR: 64% vs. 66.7% |
| TRITICC (Phase IIa, 2023) | Trifluridine/Tipiracil + Irinotecan | 28 patients with locally advanced/metastatic BTC; progressed after Gem-based chemo | PFS: ongoing (recruitment completed) | OS, ORR, DCR, and safety outcomes: ongoing |
ref https://www.esmoopen.com/article/S2059-7029(24)01476-5/fulltext
Empowering Progress in Gallbladder Cancer Treatment
The fight against gallbladder cancer requires collaborative effort and access to critical information. By providing this comprehensive and accessible data, we aim to accelerate research, foster innovation, and ultimately contribute to improved outcomes for patients worldwide. We invite researchers, clinicians, and anyone interested in GBC to explore our data hub and join us in this vital endeavor.
Feel free to submit your dataset to empower the research

